By Frank Kuo
This post originally appeared on CLEO BLOG by Frank Kuo and is reproduced with permission from its author.
Microscope, one of the most popular optical instruments, has been paving the way of biological science for the past three hundred years. With the aid of the microscope, detailed observations of sub-cell size resolution were made possible. This, in turn, accelerated our understanding of the biology in an unprecedented way. Three hundred years have passed; we now arrived at a new cross road — While triumphing on the universe of biology, a desire to develop microscopes with specificities and better resolutions is creating another revolution.
Specificities problems are less optical relevant. It is like painting different organelles of the cell with different colors. To do so, scientists use fluorescent dyes to attach to different organelles or encode them directly into the genetic codes of the proteins. So we can differentiate what they are and where they are. Scientists are quite good in doing so.
Resolution is another story. It is a barrier imposed by fundamental physics. In other words, the enemy of a microscope is diffraction, which prevents how well you can resolve two points on the focal plane. Same principle also applies to how tight you can focus a collimated beam. Using the traditional microscope, you cannot have resolution better than hundreds of nanometers if visible light is used. The axial resolution is not much better. As a result, no matter how small the particle in the focal plane is (in this case, the fluorescent dye), you would always observe a blob with some sizable volume. How do achieve better resolution? What kind of tricks scientists can play to break the diffraction limit?
For me, the first milestone in super resolution is called FIONA (Fluorescence Imaging with One Nanometer Accuracy). What a lovely name! In a nutshell, it fits the fluorescent signal with a Gaussian function. By doing so, it finds the center of the dye theoretically. Just like finding a center of the blob in the example we gave above. This method is generally adopted in modern microscopy since it localizes the location of the dye in the lateral plane quite well. There is a caveat though — you cannot have too many dyes in focal point. This is just going to screw up your fitting.
Same mathematical manipulation does not work satisfactory in axial direction. In addition to multi-photon microscopy which aims on attacking this problem, there are other neat techniques existent. The way to get around it is modifying and mixing the experimental setup with other optical phenomena. The most eye-catching technique to me is the research led by professor H. Hess in HHMI. By putting a three-way beam splitter, the florescent signal from the dye in the focal plane would interfere with itself and generate different interference pattern depending on how far the dye is offset from the true focal point. This method achieved tens of nm of axial resolution. What impresses me the most is the feeling I have when trying to understand the diagram of the experimental layout. Suddenly, you realize, the imagination to advance optical science is unlimited.
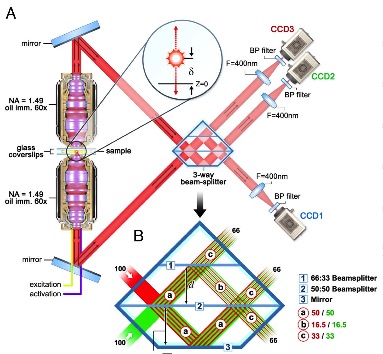
Figure 1. The optical layout for interference microscopy. Courtesy of G. Shtengel, et al. in PNAS 106 9 3125 (2009)
Continue reading »
Posted: 10 September 2011 by
Frank Kuo
| with 0 comments